Anti-cancer Compounds
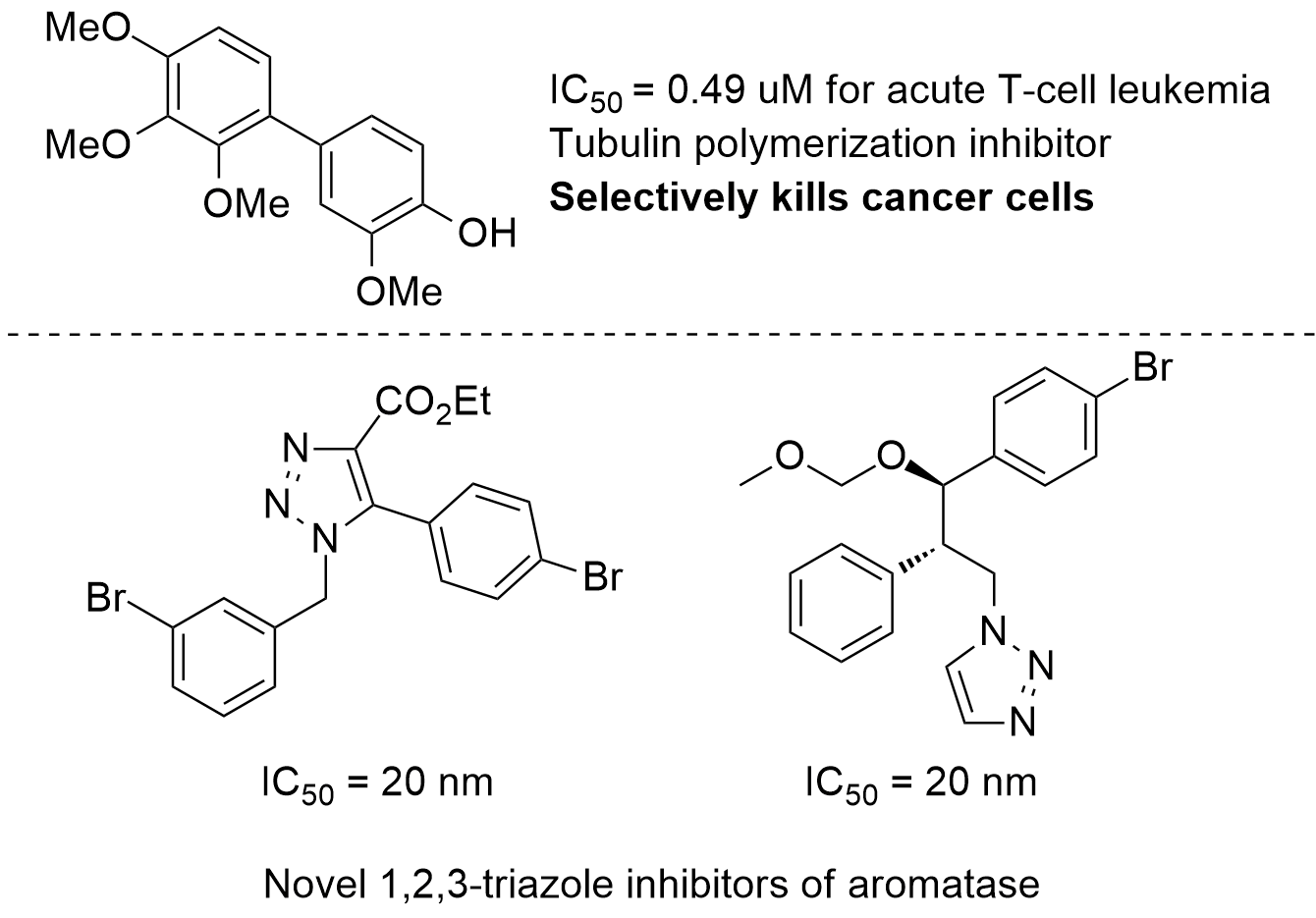
Cancer is a growing cause of death and disability worldwide. Recently, the McNulty Group has discovered a series of potent and selective inhibitors of aromatase, a clinically validated target for the treatment of estrogen receptor positive breast cancer. The compounds feature of a 1,2,3-triazole core that chelates to the heme functionality of aromatase. The activity of the compounds was also improved with the inclusion aryl-halide moieties, demonstrating that these functional groups may act like ketone bioisosteres in the active site of enzymes. Our group has perfored a substantial amount of work regarding the anti-cancer activity of certain natural products, especially the Amaryllidacea alkaloids. These alkaloids are synthetically challenging targets, and usually only available in small quantities. However, we have also discovered simple biaryl compounds that are structual analogoues of combretestatin A4 and colchicine, and share their potent anti-cancer activities. These simple compounds are readily scalable, and selectively kill cancer kills. Our group is actively pursuing novel methods and strategies for the synthesis of potent anti-cancer compounds.
|
 |
Relevant Articles Synthetic Approaches to the Naturally Occuring Anticancer Amaryllidaceae Alkaloids trans-dihydrolycoricidine and trans-dihydronarciclasine. C. Zepeda-Velázquez, J. McNulty*, Studies in Natural Products Chemistry, 53, in press (2017). Review of Cytotoxic CA4 Analogues That do Not Target Microtubules: Implications For CA4 Development. D. Tarade, S. Pandey*, J. McNulty, Mini-Rev. Med. Chem., 15, (2016), DOI: 10.2174/1389557515666160509125829. Antimitotic activity of structurally simplified biaryl analogs of the anticancer agents colchicine and combretastatin-A4. Discovery of a new class of cinnamyl-triazole as potent and selective inhibitors of aromatase. J. McNulty*, K. Keskar, A. Holloway, D. Crankshaw, Bioorg. & Med. Chem. Lett., 24, 4586-4589 (2014). Enantioselective Organocatalytic Michael-aldol Sequence to the Anticancer Natural Product (+)-trans-dihydrolycoricidine. J. McNulty*, C. Zepeda-Velazquez, Angew. Chem. Int. Ed. 53, 8450-8454 (2014). See also: Short Synthesis of a Wildflower Alkaloid, Chem. & Eng. News, July 7th, 2014, p. 28-29. See also: Synfacts, 10, 982 (2014). Investigation of aryl halides as ketone bioisosteres: refinement of potent and selective inhibitors of human cytochrome P450 19A1 (aromatase). J. McNulty*, A. J. Nielsen, C. E Brown, B. R. Difrancesco, N. Vurgun, J. J. Nair; D. Crankshaw, A. Holloway, Bioorg. & Med. Chem. Lett., 23, 6060-6063 (2013). Discovery of a novel class of aldol-derived 1,2,3-triazoles: potent and selective inhibitors of human cytochrome P450 19A1 (aromatase). J. McNulty*, J. J. Nair, N. Vurgun, B. DiFrancesco, C. Brown, A. Holloway, D. Crankshaw, B. Tsoi, Bioorg. & Med. Chem. Lett., 22, 718-722 (2012). A Novel Synthetic C1 Analog of 7-deoxypancratistatin Induces Apoptosis in p53 Positive and Negative Human Colorectal Cancer Cells by Targeting the Mitochondria: Enhancement of Activity by Tamoxifen. D. Ma, P. Tremblay, K. Mahngar, P. Akbari-Asl,J. Collins, T. Hudlicky, J. McNulty, S. Pandey*, Investig. New Drugs, 30, 1012-1027 (2012). Pancratistatin induces apoptosis and autophagy in metastatic prostate cancer cells. C. Griffin, J. McNulty, S. Pandey*, Int. J. Oncol., 38, 1549-1556 (2011). Human Cytochrome P450 Liability Studies of trans-Dihydronarciclasine: A Readily Available, Potent and Selective Cancer Cell Growth Inhibitor. J. McNulty*, A. Thorat, N. Vurgun, J. J. Nair, E. Makaji, D. J. Crankshaw, A. C. Holloway, S. Pandey, J. Nat. Prod., 74, 106-108 (2011).
|
|